NAD+ is a central molecule of cellular function and metabolism that serves many fundamental functions in the human body, particularly in cellular energy production and many cell signaling pathways critical for cellular health. NAD+ levels decrease as we get older and this decline contributes to poorer health outcomes associated with aging [1].
Nicotinamide is a form of vitamin B3 that serves various essential functions in the human body. Nicotinamide is a precursor for NAD+ synthesis that supports healthy NAD+ levels in tissues. By doing so, nicotinamide contributes to healthy cellular function, energy metabolism, DNA repair, skin health, and the overall health of our body, particularly as we age and NAD+ levels decline.
In this article, we’ll learn about the benefits of nicotinamide and how it supports general health and healthy aging.
What is Nicotinamide?
Nicotinamide, also named niacinamide, is one of the forms of vitamin B3, along with nicotinic acid (also named niacin, which is another name for vitamin B3 as well). Vitamin B3 is part of the B vitamin complex, a group of water-soluble vitamins that play important roles in cellular metabolism and energy production.
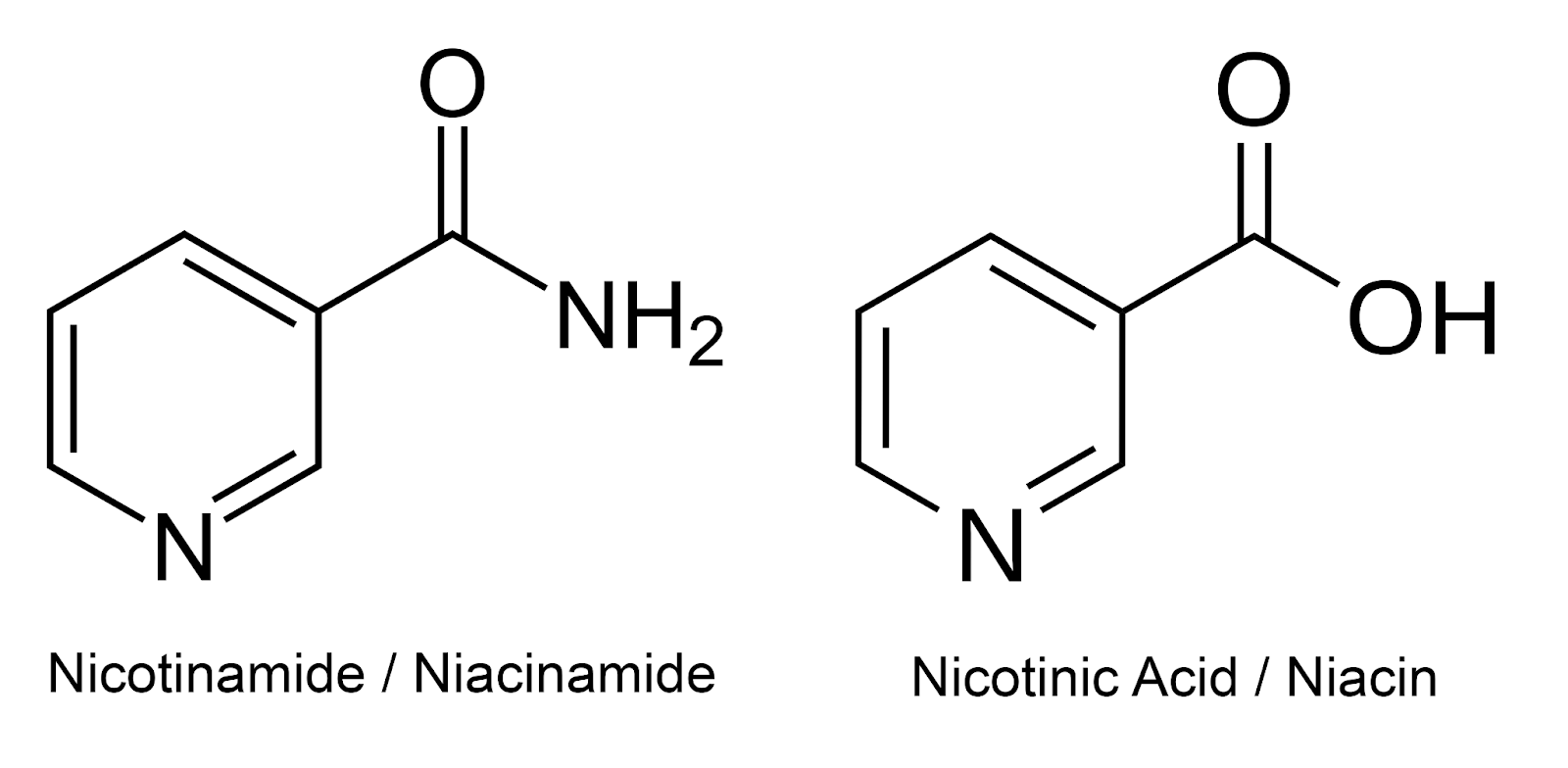
Figure 1. Structures of nicotinamide (niacinamide) and nicotinic acid (niacin).
Compounds with vitamin B3 activity are defined by their ability to contribute to the production of an important molecule called nicotinamide adenine dinucleotide (NAD). NAD is one of the coenzyme forms of vitamin B3, meaning it’s a molecule that supports the activity of enzymes. The other coenzyme form is called nicotinamide adenine dinucleotide phosphate (NADP), which also includes a nicotinamide unit in its structure. Both coenzyme forms play important roles in cellular metabolism and redox homeostasis.
Nicotinamide Is a Precursor for NAD+ Synthesis
One of the main reasons why nicotinamide is an essential molecule is because it is used to generate NAD+, which is indispensable for normal cellular functioning. Nicotinamide is actually part of the structure of NAD, which consists of an adenine molecule bound to a nicotinamide unit. NAD is found in every cell in the body mostly in two forms: NAD+, the oxidized form, and NADH, the reduced form. NAD+ and NADH have key roles in redox homeostasis—the balance between reducing and oxidizing reactions that regulates a huge number of critical biological events within cells. They are converted back and forth between these two forms in redox reactions (i.e., reactions where electron transfer occurs).

Figure 2. NAD+ ←→ NADH Redox Reaction
One of the most important functions of NAD+ is to act as an electron carrier in cellular energy pathways that produce ATP. Glycolysis (which breaks down glucose), beta-oxidation (which breaks down fatty acids), and the citric acid cycle (or Krebs cycle, which extracts electrons from carbon units derived from other pathways) produce a small amount of ATP at the cost of reducing (i.e., adding electrons) to NAD+ molecules to yield NADH (with the addition of a hydrogen proton—H+). In oxidative phosphorylation, NADH feeds those electrons into the electron transport chain where they are used to power the production of great amounts of ATP. In this process, NADH is converted back to NAD+ [2].
NAD+ also has important roles in signaling pathways and processes that are essential for cell and tissue health and general healthspan [3]. NAD+ supports stem cell function, essential for maintaining tissues’ capacity to repair and regenerate [4]. NAD+ is a co-substrate for NAD-dependent enzymes such as sirtuins, PARPs, and CD38 that mediate important processes such as metabolic adjustments, protection of mitochondrial function, redox homeostasis, DNA repair and genomic stability, apoptosis, cell differentiation, and survival, among others [4–7]. By supporting NAD+ levels, nicotinamide may support all these actions.
How Nicotinamide Works to Support NAD+
NAD+ and NADH must be maintained at a high NAD+/NADH ratio (i.e., higher levels of NAD+ than NADH) to sustain cellular energy metabolism and healthy mitochondrial and cellular function [2,4,8].
To maintain a high NAD+/NADH ratio, NAD+ must be continuously synthesized, metabolized, and recycled in the cell. It is so important that cells have multiple strategies to replenish NAD+.
It is important that cells have multiple strategies to replenish NAD+.
NAD+ that is reduced to NADH in cell energy pathways is quickly regenerated as ATP is produced, allowing cells to maintain the NAD+/NADH ratio. But NAD+-dependent signaling reactions, on the other hand, actually break apart the NAD+ molecule, releasing nicotinamide as a byproduct [9]. To maintain a high NAD+/NADH ratio, nicotinamide is recycled back to NAD+ through one of the pathways of NAD+ production, which is known as the salvage pathway (because it salvages nicotinamide).
In the salvage pathway, nicotinamide is converted into NAD+ in two steps with nicotinamide mononucleotide (NMN) as an intermediate (nicotinamide → NMN → NAD+). NAD+ can then be consumed in NAD+-dependent signaling reactions, regenerating nicotinamide in the process. Therefore, the salvage pathway is a cycle.
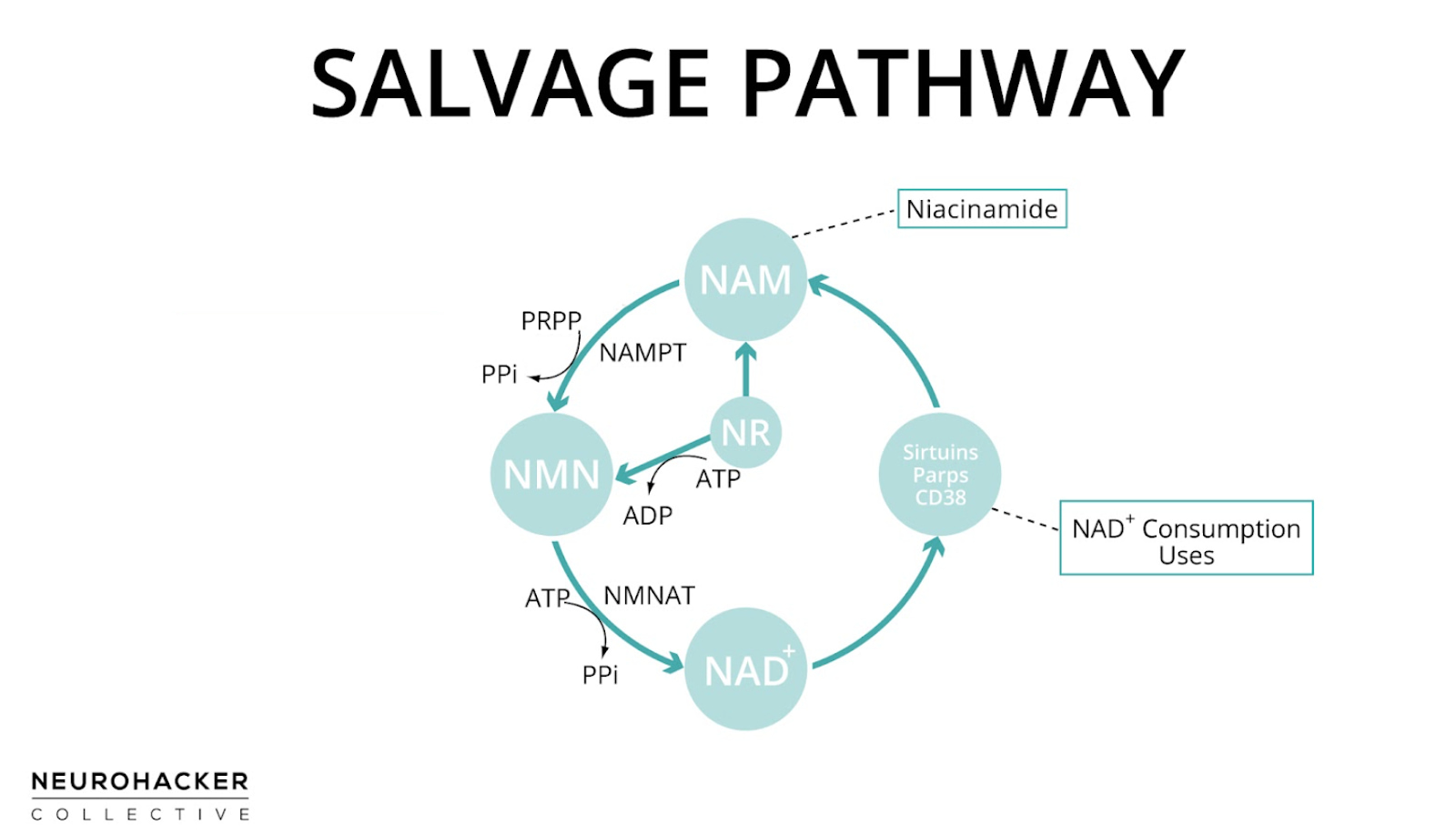
Figure 3. Salvage pathway of NAD+ production
But it’s not just nicotinamide generated from NAD+ consumption that is used in the salvage pathway—nicotinamide molecules from foods or supplements are also used to generate NAD+ through the same pathway. NMN and nicotinamide riboside (NR) are two other NAD+ biosynthesis precursors that can enhance NAD+ levels through the salvage pathway. NMN is an intermediate of this pathway, but NR is not; NR enters the salvage pathway by being metabolized to NMN or niacinamide, which can then yield NAD+ [10].
In addition to the salvage pathway, cells can also produce NAD+ from nicotinic acid (Preiss-Handler pathway) and the amino acid tryptophan (de novo synthesis pathway).
Supporting NAD+ Levels With Aging
Maintaining adequate levels of NAD+ is crucial for maintaining good health. As we age, NAD+ levels in tissues decline [11–13]. This reduction in NAD+ availability is believed to play a great part in age-related physiological decline and to be associated with declines in mental and physical performance [14,15].
These are several characteristics of aging that are shared by all organisms. These are known as the hallmarks of aging, and there are twelve [16]. Several of the hallmarks are known to be directly impacted by NAD+ availability, namely mitochondrial dysfunction, genomic instability, deregulated nutrient-sensing, and stem cell exhaustion. It has even been proposed that declines in NAD+ may contribute to all of the hallmarks of aging [17].
Several of the hallmarks of aging are known to be directly impacted by NAD+ availability, namely mitochondrial dysfunction, genomic instability, deregulated nutrient-sensing, and stem cell exhaustion.
Although lower levels of NAD+ are characteristic of aging, some people have suboptimal NAD+ levels even at younger ages. NAD+ levels may start to decline by around thirty years of age. Most people in their 40s have comparatively low levels of NAD+ relative to a healthy 20-year-old and the decline keeps accelerating with each passing decade [11,18]. The decline in NAD+ with age is so consistent that it has been proposed as a biomarker of aging [19–21].
Studies with aged animals have shown that restoration of NAD+ levels mitigates many age-related changes by enhancing mitochondrial function and energy metabolism, promoting DNA repair, restoring cellular defenses against oxidative stress, and modulating cellular senescence. By doing so, restoring NAD+ can ameliorate systemic and tissue dysfunction and promote healthspan [22,23].
Supplementation with nicotinamide in amounts substantially greater than the recommended daily allowance (RDA) can help to enhance NAD+ levels [24–27]. In animals studies, nicotinamide administration markedly increased NAD+ levels in several tissues, including the brain (nicotinamide crosses the blood-brain barrier) [28–39], eyes [40], blood [26,41–45], kidney [46], liver [42,43,47–57], skeletal muscle [58], bone marrow [59], lungs [60], mammary tissue [49,61], pancreas [62], thyroid [63] and testes [64]. Because NAD+ is so ubiquitous in the body and essential for the health of all tissues, the support of NAD+ levels by nicotinamide may manifest as many diverse health benefits.
Other Uses and Benefits of Nicotinamide
Supporting NADP Synthesis
It’s not just NAD+ that’s important. Nicotinamide is also a precursor of NADP, which like NAD, exists in an oxidized (NADP+) and a reduced (NADPH) form. NADPH is a cofactor for several enzymes involved in redox reactions with important functions [65], including cytochrome P450 enzymes that detoxify xenobiotics [66] or glutathione reductase, which maintains the levels of reduced glutathione, an important antioxidant molecule that confers protection against oxidative stress [67].
NADPH is also used in many anabolic reactions (i.e., metabolic reactions that construct macromolecules from smaller units), including the synthesis of fatty acids, cholesterol, and DNA [68].
Skin Health
An additional benefit of nicotinamide is the support of skin biology and health by restoring cellular NAD+ pools and facilitating efficient metabolism and ATP synthesis [69].
In studies in vitro, nicotinamide restored NAD+/NADH levels in aged fibroblasts youthful levels [70]. Fibroblasts are the main cells of the dermis and are responsible for the production of structural molecules that are responsible for the skin’s mechanical properties, such as elasticity and firmness. Nicotinamide also restored mitochondrial integrity in aged human fibroblasts [71]. Furthermore, nicotinamide supported skin cells’ capacity to protect themselves from oxidative stress and environmental stressors, which are the main causes of skin aging [72–76].
Most human studies have used nicotinamide applied topically to the skin. These have shown that nicotinamide supports skin appearance, lightness, uniform pigmentation, and elasticity [77–80]. Supplementation with oral nicotinamide s has supported uniform skin pigmentation [79].
Dietary Sources of Nicotinamide
Vitamins are natural compounds that our body needs in small amounts for proper cellular metabolic function and good health. Vitamins are essential nutrients because they cannot be synthesized in the body in sufficient amounts for survival, and must therefore be obtained through the diet, either from foods or from dietary supplements.
Vitamin B3 can be obtained from the diet in different forms. Niacin equivalents (or vitamin B3 equivalents) is a term used to describe all dietary molecules that can contribute to vitamin B3 status in the body. These include nicotinamide, niacin, NMN, NR, NAD, and NADP.
Niacin equivalents are found in all dietary animal, plant, and fungal foods (like humans these organisms require NAD for life). Meat, eggs, fish, dairy, some vegetables, and whole grains are considered good sources of vitamin B3, but the form and bioavailability of niacin equivalents can differ substantially.
In animal-based foods, niacin equivalents occur mostly as NAD and NADP, which have high bioavailability [81]. These molecules are enzymatically broken down during digestion into nicotinamide, which is the form that is then absorbed [82,83]. Red meat, chicken, and fish are among the richest sources of nicotinamide [84,85].
Nicotinic acid is the main form of vitamin B3 in plant foods, followed by nicotinamide. In some cereal grains, nicotinic acid is bound to polysaccharides and glycopeptides, forming a complex known as niacytin, which is undigestible by humans and thus poorly bioavailable [84,85]. Niacytin is concentrated in the outer layers of whole grains, which are removed by milling, which is why whole grains are a better source of vitamin B3.
Overall, nicotinamide is the main form of vitamin B3 absorbed from food sources [86–90]. It is also the primary form released into the blood for distribution to other tissues because, regardless of what precursor is used to synthesize NAD+ in the liver, nicotinamide is ultimately produced when NAD+ is used in NAD+-dependent reactions. Nicotinamide is believed to be the preferred precursor for making NAD molecules in most peripheral tissues [58,91,92].
Nicotinamide in Qualia Supplements
Nicotinamide (or niacinamide) and nicotinic acid (or niacin) have been used for decades in supplements and are the most common supplemental forms of vitamin B3. A great advantage of nicotinamide over nicotinic acid as a supplement is that nicotinamide typically does not cause flushing, while nicotinic acid typically does. Many of the other side effects observed for nicotinic acid, such as headache, rash, and dizziness, also occur much less frequently with nicotinamide and only at much higher amounts [85,93,94].
Both nicotinamide and nicotinic acid enhance NAD+ levels [95]. Their actions are complementary because they yield NAD+ through different pathways. The NAD+ molecule is so important that cells evolved multiple ways to create it. Cells in some tissues rely preferably on one pathway, but other tissues may use different pathways of NAD+ production to varying extents to meet changing NAD+ needs. So rather than supporting only one pathway of NAD+ production, we support different ways to make it.
That’s why we have nicotinamide and nicotinic acid forms in our healthy aging line products Qualia Life and Qualia NAD+. In fact, in Qualia Life, in addition to nicotinamide and nicotinic acid, we also have L-Tryptophan, so it supports all three pathways of NAD+ synthesis. In Qualia NAD+, in addition to nicotinamide and nicotinic acid, we also have another form known to enhance NAD+ levels: NIAGEN® Nicotinamide Riboside (NR) [96–100].
*These statements have not been evaluated by the Food and Drug Administration. This product is not intended to diagnose, treat, cure, or prevent any disease.
References
[1]A.J. Covarrubias, R. Perrone, A. Grozio, E. Verdin, Nat. Rev. Mol. Cell Biol. 22 (2021) 119–141.
[2]D.L. Nelson, M.M. Cox, Lehninger Principles of Biochemistry, 7th Edition, W. H. Freeman and Company, 2017.
[3]A.R. Mendelsohn, J.W. Larrick, Rejuvenation Res. 20 (2017) 244–247.
[4]H. Zhang, D. Ryu, Y. Wu, K. Gariani, X. Wang, P. Luan, D. D’Amico, E.R. Ropelle, M.P. Lutolf, R. Aebersold, K. Schoonjans, K.J. Menzies, J. Auwerx, Science 352 (2016) 1436–1443.
[5]M. Pittelli, R. Felici, V. Pitozzi, L. Giovannelli, E. Bigagli, F. Cialdai, G. Romano, F. Moroni, A. Chiarugi, Mol. Pharmacol. 80 (2011) 1136–1146.
[6]Z. Herceg, Z.Q. Wang, Mutat. Res. 477 (2001) 97–110.
[7]R.H. Houtkooper, E. Pirinen, J. Auwerx, Nat. Rev. Mol. Cell Biol. 13 (2012) 225–238.
[8]L. Mouchiroud, R.H. Houtkooper, N. Moullan, E. Katsyuba, D. Ryu, C. Cantó, A. Mottis, Y.-S. Jo, M. Viswanathan, K. Schoonjans, L. Guarente, J. Auwerx, Cell 154 (2013) 430–441.
[9]A.P. Gomes, N.L. Price, A.J.Y. Ling, J.J. Moslehi, M.K. Montgomery, L. Rajman, J.P. White, J.S. Teodoro, C.D. Wrann, B.P. Hubbard, E.M. Mercken, C.M. Palmeira, R. de Cabo, A.P. Rolo, N. Turner, E.L. Bell, D.A. Sinclair, Cell 155 (2013) 1624–1638.
[10]J. Yoshino, J.A. Baur, S.-I. Imai, Cell Metab. 27 (2018) 513–528.
[11]J. Clement, M. Wong, A. Poljak, P. Sachdev, N. Braidy, Rejuvenation Res. (2018).
[12]S.-I. Imai, L. Guarente, Trends Cell Biol. 24 (2014) 464–471.
[13]E. Verdin, Science 350 (2015) 1208–1213.
[14]M.C. Dalmasso, M. Arán, P. Galeano, S. Perin, P. Giavalisco, P.V. Martino Adami, G.V. Novack, E.M. Castaño, A.C. Cuello, M. Scherer, W. Maier, M. Wagner, S. Riedel-Heller, A. Ramirez, LMorelli, Front Mol Biosci 9 (2022) 1067296.
[15]A. Karas, D. Holmannova, P. Borsky, Z. Fiala, C. Andrys, K. Hamakova, T. Svadlakova, V. Palicka, J. Krejsek, V. Rehacek, M. Esterkova, H. Kovarikova, L. Borska, Biomedicines 10 (2022).
[16]C. López-Otín, M.A. Blasco, L. Partridge, M. Serrano, G. Kroemer, Cell 186 (2023) 243–278.
[17]Y. Aman, Y. Qiu, J. Tao, E.F. Fang, Translational Medicine of Aging 2 (2018) 30–37.
[18]H. Massudi, R. Grant, N. Braidy, J. Guest, B. Farnsworth, G.J. Guillemin, PLoS One 7 (2012) e42357.
[19]T. Jayasena, S. Bustamante, J. Clement, R. Welschinger, G.A. Caplan, P.S. Sachdev, N. Braidy, Methods Mol. Biol. 2138 (2020) 207–216.
[20]R. Sharma, A. Ramanathan, Proteomics 20 (2020) e1800407.
[21]R. Furrer, C. Handschin, J. Physiol. 601 (2023) 2057–2068.
[22]C.F. Lee, A. Caudal, L. Abell, G.A. Nagana Gowda, R. Tian, Sci. Rep. 9 (2019) 3073.
[23]E.F. Fang, S. Lautrup, Y. Hou, T.G. Demarest, D.L. Croteau, M.P. Mattson, V.A. Bohr, Trends Mol. Med. 23 (2017) 899–916.
[24]T.K. Ito, T. Sato, Y. Takanashi, Z. Tamannaa, T. Kitamoto, K. Odagiri, M. Setou, Translational Medicine of Aging 5 (2021) 43–51.
[25]T.K. Ito, T. Sato, A. Hakamata, Y. Onoda, S. Sato, F. Yamazaki, M. Horikawa, Y. Takahashi, T. Kitamoto, M. Suzuki, S. Uchida, K. Odagiri, M. Setou, Translational Medicine of Aging 4 (2020) 45–54.
[26]Y. Takahashi, A. Tanaka, T. Nakamura, T. Fukuwatari, K. Shibata, N. Shimada, I. Ebihara, H. Koide, Kidney Int. 65 (2004) 1099–1104.
[27]T. Fukuwatari, K. Shibata, J. Nutr. Sci. Vitaminol. 55 (2009) 279–281.
[28]R. Spector, Neurochem. Res. 12 (1987) 27–31.
[29]L.K. Klaidman, S.K. Mukherjee, T.P. Hutchin, J.D. Adams, Neurosci. Lett. 206 (1996) 5–8.
[30]F.J. Wan, H.C. Lin, B.H. Kang, C.J. Tseng, C.S. Tung, Brain Res. Bull. 50 (1999) 167–171.
[31]J. Yang, L.K. Klaidman, A. Nalbandian, J. Oliver, M.L. Chang, P.H. Chan, J.D. Adams Jr, Neurosci. Lett. 333 (2002) 91–94.
[32]F. Sadanaga-Akiyoshi, H. Yao, S.-I. Tanuma, T. Nakahara, J.S. Hong, S. Ibayashi, H. Uchimura, M. Fujishima, Neurochem. Res. 28 (2003) 1227–1234.
[33]L. Klaidman, M. Morales, S. Kem, J. Yang, M.-L. Chang, J.D. Adams Jr, Pharmacology 69 (2003) 150–157.
[34]R. Spector, C.E. Johanson, J. Neurochem. 103 (2007) 425–438.
[35]D. Liu, R. Gharavi, M. Pitta, M. Gleichmann, M.P. Mattson, Neuromolecular Med. 11 (2009) 28–42.
[36]C.S. Siegel, L.D. McCullough, Neuroscience 237 (2013) 223–231.
[37]J. Li, X. Dou, S. Li, X. Zhang, Y. Zeng, Z. Song, Biochim. Biophys. Acta 1853 (2015) 2929–2936.
[38]F. Li, T. Fushima, G. Oyanagi, H.W.D. Townley-Tilson, E. Sato, H. Nakada, Y. Oe, J.R. Hagaman, J. Wilder, M. Li, A. Sekimoto, D. Saigusa, H. Sato, S. Ito, J.C. Jennette, N. Maeda, S.A. Karumanchi, O. Smithies, N. Takahashi, Proc. Natl. Acad. Sci. U. S. A. 113 (2016) 13450–13455.
[39]C. Wang, Y. Zhang, J. Ding, Z. Zhao, C. Qian, Y. Luan, G.-J. Teng, Neural Plast. 2017 (2017) 7019803.
[40]P.A. Williams, J.M. Harder, N.E. Foxworth, K.E. Cochran, V.M. Philip, V. Porciatti, O. Smithies, S.W.M. John, Science 355 (2017) 756–760.
[41]B. Petrack, P. Greengard, H. Kalinsky, J. Biol. Chem. 241 (1966) 2367–2372.
[42]T.M. Jackson, J.M. Rawling, B.D. Roebuck, J.B. Kirkland, J. Nutr. 125 (1995) 1455–1461.
[43]M.M. ApSimon, J.M. Rawling, J.B. Kirkland, J. Nutr. 125 (1995) 1826–1832.
[44]K. Majamaa, H. Rusanen, A.M. Remes, J. Pyhtinen, I.E. Hassinen, Life Sci. 58 (1996) 691–699.
[45]K. Majamaa, H. Rusanen, A. Remes, I.E. Hassinen, Mol. Cell. Biochem. 174 (1997) 291–296.
[46]M.T. Tran, Z.K. Zsengeller, A.H. Berg, E.V. Khankin, M.K. Bhasin, W. Kim, C.B. Clish, I.E. Stillman, S.A. Karumanchi, E.P. Rhee, S.M. Parikh, Nature 531 (2016) 528–532.
[47]R. Van Reen, M. Quesada, Biochem. Biophys. Res. Commun. 24 (1966) 56–60.
[48]O. Hayaishi, H. Ijichi, A. Ichiyama, Adv. Enzyme Regul. 5 (1967) 9–22.
[49]S. Pinder, A.L. Greenbaum, Biochem. J 102 (1967) 20C–21C.
[50]J.B. Clark, S. Pinder, Biochem. J 114 (1969) 321–330.
[51]J.T. MacGregor, A. Burkhalter, Biochem. Pharmacol. 22 (1973) 2645–2658.
[52]C.L. Baum, J. Selhub, I.H. Rosenberg, Biochem. J 204 (1982) 203–207.
[53]G.M. McCreanor, D.A. Bender, Br. J. Nutr. 56 (1986) 577–586.
[54]V. Batra, B. Kislay, Mutat. Res./Fundam. Mol. Mech. Mutag. 749 (2013) 28–38.
[55]S.J. Yang, J.M. Choi, L. Kim, S.E. Park, E.J. Rhee, W.Y. Lee, K.W. Oh, S.W. Park, C.-Y. Park, J. Nutr. Biochem. 25 (2014) 66–72.
[56]S.A.J. Trammell, M.S. Schmidt, B.J. Weidemann, P. Redpath, F. Jaksch, R.W. Dellinger, Z. Li, E.D. Abel, M.E. Migaud, C. Brenner, Nat. Commun. 7 (2016) 12948.
[57]S.Á. Mejía, L.A.B. Gutman, C.O. Camarillo, R.M. Navarro, M.C.S. Becerra, L.D. Santana, M. Cruz, E.H. Pérez, M.D. Flores, Eur. J. Pharmacol. 818 (2018) 499–507.
[58]K. Shibata, T. Hayakawa, K. Iwai, Agric. Biol. Chem. 50 (1986) 3037–3041.
[59]A.C. Boyonoski, J.C. Spronck, R.M. Jacobs, G.M. Shah, G.G. Poirier, J.B. Kirkland, J. Nutr. 132 (2002) 115–120.
[60]A. Nagai, H. Matsumiya, M. Hayashi, S. Yasui, H. Okamoto, K. Konno, Exp. Lung Res. 20 (1994) 263–281.
[61]A.L. Greenbaum, S. Pinder, Biochem. J 107 (1968) 55–62.
[62]H. Tjälve, E. Wilander, Acta Endocrinol. 83 (1976) 357–364.
[63]C.H. Bastomsky, V. Abbassi, J.M. McKenzie, Endocrinology 83 (1968) 79–85.
[64]L.F. Lin, L.M. Henderson, J. Biol. Chem. 247 (1972) 8023–8030.
[65]A.A. Sauve, J. Pharmacol. Exp. Ther. 324 (2008) 883–893.
[66]A.V. Pandey, C.E. Flück, Pharmacol. Ther. 138 (2013) 229–254.
[67]G. Filomeni, G. Rotilio, M.R. Ciriolo, Biochem. Pharmacol. 64 (2002) 1057–1064.
[68]N. Pollak, C. Dölle, M. Ziegler, Biochem. J 402 (2007) 205–218.
[69]J.E. Oblong, DNA Repair 23 (2014) 59–63.
[70]R. Osborne, R.S. Carver, L.A. Mullins, D.R. Finlay, Br. J. Dermatol. 169 Suppl 2 (2013) 32–38.
[71]J.E. Oblong, A. Bowman, H.A. Rovito, B.B. Jarrold, J.D. Sherrill, M.R. Black, G. Nelson, A.B. Kimball, M.A. Birch-Machin, Aging Cell (2020) e13248.
[72]H.A. Rovito, J.E. Oblong, Br. J. Dermatol. 169 Suppl 2 (2013) 15–24.
[73]J. Park, G.M. Halliday, D. Surjana, D.L. Damian, Photochem. Photobiol. 86 (2010) 942–948.
[74]B.C. Thompson, G.M. Halliday, D.L. Damian, PLoS One 10 (2015) e0117491.
[75]G. Monfrecola, F. Gaudiello, T. Cirillo, G. Fabbrocini, A. Balato, S. Lembo, Clin. Exp. Dermatol. 38 (2013) 185–188.
[76]J.C. Bierman, T. Laughlin, M. Tamura, B.C. Hulette, C.E. Mack, J.D. Sherrill, C.Y.R. Tan, M. Morenc, S. Bellanger, J.E. Oblong, Int. J. Cosmet. Sci. 42 (2020) 501–511.
[77]D.L. Bissett, J.E. Oblong, C.A. Berge, Dermatol. Surg. 31 (2005) 860–5; discussion 865.
[78]A. Kawada, N. Konishi, N. Oiso, S. Kawara, A. Date, J. Dermatol. 35 (2008) 637–642.
[79]T. Hakozaki, L. Minwalla, J. Zhuang, M. Chhoa, A. Matsubara, K. Miyamoto, A. Greatens, G.G. Hillebrand, D.L. Bissett, R.E. Boissy, Br. J. Dermatol. 147 (2002) 20–31.
[80]Z.D. Draelos, A. Matsubara, K. Smiles, J. Cosmet. Laser Ther. 8 (2006) 96–101.
[81]L.M. Henderson, Annu. Rev. Nutr. 3 (1983) 289–307.
[82]C.J. Gross, L.M. Henderson, J. Nutr. 113 (1983) 412–420.
[84]L.J. Hill, A.C. Williams, Int. J. Tryptophan Res. 10 (2017) 1178646917704662.
[85]U.S. National Institutes of Health - Office of Dietary Supplements (2015).
[86]J.B. Turner, D.E. Hughes, Exp. Physiol. 47 (1962) 107–123.
[87]C. Streffer, J. Benes, Eur. J. Biochem. 21 (1971) 357–362.
[88]P.B. Collins, S. Chaykin, J. Biol. Chem. 247 (1972) 778–783.
[89]L.M. Henderson, C.J. Gross, J. Nutr. 109 (1979) 654–662.
[90]L.M. Henderson, C.J. Gross, J. Nutr. 109 (1979) 646–653.
[91]K.L. Bogan, C. Brenner, Annu. Rev. Nutr. 28 (2008) 115–130.
[92]S.-I. Imai, FEBS Lett. 585 (2011) 1657–1662.
[93]Scientific Committee on Food and Scientific Panel on Dietetic Products Nutrition and Allergies, Tolerable Upper Intake Levels For Vitamins And Minerals. European Food Safety Authority, European Food Safety Authority, 2006.
[94]M. Knip, I.F. Douek, W.P. Moore, H.A. Gillmor, A.E. McLean, P.J. Bingley, E.A. Gale, European Nicotinamide Diabetes Intervention Trial Group, Diabetologia 43 (2000) 1337–1345.
[95]X. Li, H. Yang, H. Jin, H. Turkez, G. Ozturk, H.L. Doganay, C. Zhang, J. Nielsen, M. Uhlén, J. Borén, A. Mardinoglu, Free Radic. Biol. Med. 205 (2023) 77–89.
[96]S.E. Airhart, L.M. Shireman, L.J. Risler, G.D. Anderson, G.A. Nagana Gowda, D. Raftery, R. Tian, D.D. Shen, K.D. O’Brien, PLoS One 12 (2017) e0186459.
[97]C.R. Martens, B.A. Denman, M.R. Mazzo, M.L. Armstrong, N. Reisdorph, M.B. McQueen, M. Chonchol, D.R. Seals, Nat. Commun. 9 (2018) 1286.
[98]Y.S. Elhassan, K. Kluckova, R.S. Fletcher, M.S. Schmidt, A. Garten, C.L. Doig, D.M. Cartwright, L. Oakey, C.V. Burley, N. Jenkinson, M. Wilson, S.J.E. Lucas, I. Akerman, A. Seabright, Y.-C. Lai, D.A. Tennant, P. Nightingale, G.A. Wallis, K.N. Manolopoulos, C. Brenner, A. Philp, G.G. Lavery, Cell Rep. 28 (2019) 1717–1728.e6.
[99]D. Conze, C. Brenner, C.L. Kruger, Sci. Rep. 9 (2019) 9772.
[100]H.A.K. Lapatto, M. Kuusela, A. Heikkinen, M. Muniandy, B.W. van der Kolk, S. Gopalakrishnan, N. Pöllänen, M. Sandvik, M.S. Schmidt, S. Heinonen, S. Saari, J. Kuula, A. Hakkarainen, J. Tampio, T. Saarinen, M.-R. Taskinen, N. Lundbom, P.-H. Groop, M. Tiirola, P. Katajisto, M. Lehtonen, C. Brenner, J. Kaprio, S. Pekkala, M. Ollikainen, K.H. Pietiläinen, E. Pirinen, Sci Adv 9 (2023) eadd5163.
[101]S. Brady, G. Siegel, R. Wayne Albers, D. Price, Basic Neurochemistry: Principles of Molecular, Cellular, and Medical Neurobiology, Academic Press, 2011.



No Comments Yet
Sign in or Register to Comment